Why Spike Protein Causes Abnormal, Foot-Long Blood Clots, 200 Symptoms
Spike proteins don’t only activate epithelial cells (EC) and promote localized inflammation. They also promote systemic inflammation as ACE2 is almost everywhere inside our major organs and tissues.
Consequently, more pro-inflammatory genes are expressed. More and more immune cells are attracted and sent to the injured or infected tissues (vessels in the lung, heart, gut, etc).
A number of subsequent events collectively contribute to the clotting cascade:
- Complement-mediated inflammation of epitheliums (endothelialitis): Spike proteins docking on ACE2 ECs activates the complement pathway and coagulation cascade, resulting in a systemic endothelialitis (lung injury) and procoagulant state (tendency to develop blood clots).
- As the complement destroys the endothelium, the procoagulant von Willebrand factor (vWF) and FVIII are released. A significant increase of vWF can form multimers that promote thrombus growth. vWF is secreted mainly from endothelial cells and from a-granules of platelets (megakaryocytes derived). This is comparable to the string in the “beads and string” of a necklace where the beads represent platelets.
- Platelet storm: Platelets are a small fragment of the megakaryocytes. The complement anaphylatoxins C3a and C5a activate platelets and increase the production of tissue factor further promoting a clotting forming state.
ACE2 receptors are present on platelets, and this may contribute to the massive platelet aggregation, which is a characteristic of severe COVID-19 infection. - Activation of neutrophils leads to formation of neutrophil extracellular traps (NETs), a process sometimes referred to as NETosis, which contributes to thrombosis.
- EC injury is compounded by toll-like receptor (TLR) activation by viral RNA recognition, with resulting increased reactive oxidative species (ROS) production. The increased ROS further upregulates the expression of vWF.
- Spike protein can induce expression and secretion of a series of clotting proteins which cascades into the clotting process, including factor (F)-V, thrombin, and fibrinogen to promote clotting process.
Spike Protein Dysregulates RAAS, Worsening the Clotting State
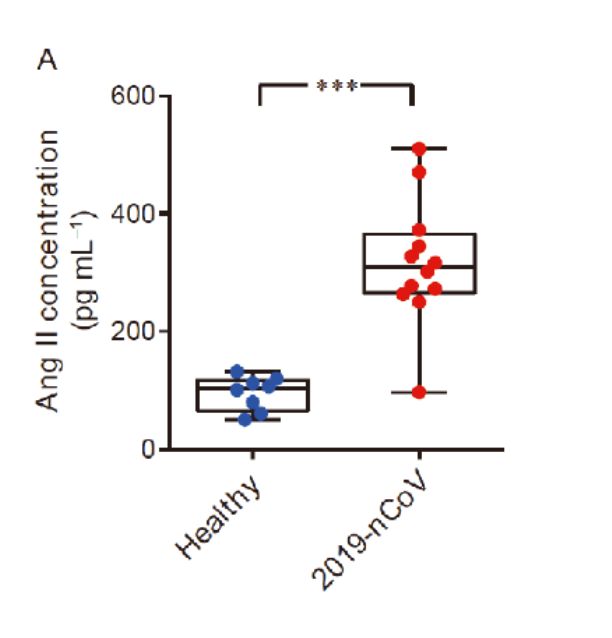
Due to the spike protein directly interacting with ACE2 expression, COVID-19 patients showed an elevated level of serum angiotensin II indicating a dysregulation of the RAA system (renin angiotensin aldosterone system, or RAAS).
Traditionally, people think angiotensin II is a neurohormone that stimulates the constriction of vascular smooth muscle cells and is responsible for salt and water balance.
However, there have been abundant studies supporting the idea that angiotensin II is capable of initiating and upregulating inflammatory responses, worsening the clotting state.
In a regulated and self-limited immune response, these mechanisms help to calm down the local injury, with subsequent healing and returning to a resting EC state.
However, for predisposed COVID-19 patients or vaccinated people, the factors strengthening clot formation are way heavier than healing mechanisms, all of which lead to an escalating thrombotic cascade.
Here is a short summary of the first scene of the clot story: spike induced endothelial disruption, massive amounts of vWF released, a subsequent platelet storm, hypoxia induced upregulation and activation of vWF, fibrous network from neutrophil extracellular traps (NETs), as well as increased angiotensin II level, all adding up to initiate thrombogenesis.
This is how the clotting mechanism comes to be. Furthermore, the upcoming second scene takes another pivotal part in the whole story.
A COVID vaccine instructs the cells to produce large quantities of spike proteins. Normal biochemical and physiological processes are “hijacked” in order to make an abnormal amount of these spike proteins.
These abnormal amounts of spike protein have more surprising direct effects on clots.
Spike Proteins Directly Disrupt the Clot Dissolving Mechanism
In a normal healthy person, the body will, in the presence of a blood clot, break it down by a process of fibrinolysis. This is a natural healing and balancing mechanism to prevent an abundance of blood clots.
During this process, Tissue Plasminogen activator (TPA, coming from the endothelium) helps plasminogen change into plasmin and then generate d-dimer (a small protein fragment left when a blood clot dissolves).
It has been discovered that fibrinogen in blood can clot into an abnormal “amyloid” form of fibrin that (like other β-rich amyloids and prions) is relatively resistant to proteolysis (fibrinolysis).
This is strongly manifested in the platelet-poor plasma (PPP) of individuals with long COVID. The extensive fibrin amyloid microclots can persist.
In an outstanding study by Grobbelaar published in Bioscience Reports in August 2021, the biomarker S1 (or the intruding part of the spike protein) alone can induce fibrin resistance to fibrinolysis, leading to unopposed microclot formation.
When spike protein S1 was added to healthy PPP, it resulted in structural changes to β and γ fibrin(ogen), complement 3, and prothrombin. These proteins were substantially resistant to trypsinization in the presence of spike protein S1.
Hence, the results suggest that the presence of spike protein in circulation may contribute to the hyper clotting status, and may cause substantial impairment of the clot dissolving process.
Such lytic impairment may result in the persistent large microclots that people have reported and which have been found in plasma samples of COVID-19 patients.
These microclots block up capillaries, and thus to limit the passage of red blood cells, and hence oxygen exchange, which can actually underpin the majority of these symptoms.
Spike Proteins Form Amyloid-Like Substance
Furthermore, to everyone’s surprise again, spike proteins are identified to present seven amyloidogenic sequences and are able to form amyloid-like substances.
In other words, these spike proteins are similar to those beta-amyloid or tau or alpha-synuclein like substances which may cause neuronal loss in Alzheimer’s or Parkinson’s disease patients.
Their structure makes it easy to form tighter string-like bonded structures with longitudinal twisting as well as cross binding, forming a fibrous-like structure visible under the microscope.
Researchers have found that plasma samples from long COVID patients still contain large anomalous (amyloid) deposits (microclots), which are resistant to fibrinolysis (compared to plasma from controls and diabetes), even after trypsinization (cell dissociation after an enzyme breaks down proteins).
After a second trypsinization, the persistent pellet deposits (microclots) were solubilized. Various inflammatory molecules substantially increased in both the supernatant and trapped in the solubilized pellet deposits of COVID-19, versus that of the control samples.
Of particular interest was a substantial increase in α(2)-antiplasmin (α2AP), various fibrinogen chains, as well as Serum Amyloid A (SAA) that were trapped in the solubilized fibrinolytic-resistant pellet deposits.
Significant abnormal amyloid microclot formation that are resistant to fibrinolysis, increased α2AP, and the surge of acute phase inflammatory molecules may therefore be central contributors in both COVID-19 infection and as well as COVID vaccine-related syndrome.
Spike Protein Inhibits Another Anti-Clot Mechanism
Spike protein is just one surprise after another.
It’s been reported that the spike protein can competitively inhibit the bindings of antithrombin and heparin cofactor II to heparin, causing abnormal increase in thrombin (clotting) activity.
SARS-CoV-2 spike proteins at a similar concentration (~10 μg/mL) as the viral load in critically ill patients can directly cause blood coagulation and thrombosis in the zebrafish model.
To summarize, these unexpected negative effects of spike protein on the dissolving process of blood clots, plus its amyloid nature, all may be the key contributory factors to the abnormal, lengthy fibrous clots observed in COVID-related conditions.

No comments:
Post a Comment